Introduction: Antibioprophylaxis in patients with cancer and neutropenia is controversial and is not a broadly recommended intervention in management of patients with hematological malignancies. Previous study showed that prophylactic treatment with levofloxacin (LEVO) is an effective and well-tolerated strategy to prevent febrile episodes and other relevant infection-related outcomes in patients with cancer and neutropenia, including acute leukemia ( Bucaneve et al., NEJM 2005). However, impact on microbial resistance discouraged the widespread use of this strategy., Venetoclax (VEN) and azacitidine (AZA) combination was recently approved in newly diagnosed patients with AML who are ineligible for intensive chemotherapy. VEN-AZA allowed higher rates of complete remission and longer overall survival but twice the incidence of febrile neutropenia compared to AZA alone making primary antibioprophylaxis a relevant question, especially in patients who are treated as outpatients or discharged after the seven days of AZA treatment.
Methods: We retrospectively selected AML patients who received VEN-AZA treatment from the Toulouse-Bordeaux DATAML registry. The two centers have similar management protocols, but patients in Toulouse (but not in Bordeaux) receive antibacterial prophylaxis with LEVO 500 mg/day from day 11 during the neutropenia period following the 1 st course of VEN-AZA, until neutrophil recovery (>0.5 G/l). We included ≥ 18-year-old non-APL de novo or secondary AML receiving VEN-AZA treatment between June 2015 and May 2023, excluding patients that deceased before day 11 VEN-AZA or who were receiving antibiotherapy before start of VEN-AZA. The primary endpoint was the incidence of febrile neutropenia during the first course of VEN-AZA. Secondary endpoints included clinically documented infections, microbiologically documented infections, and safety data.
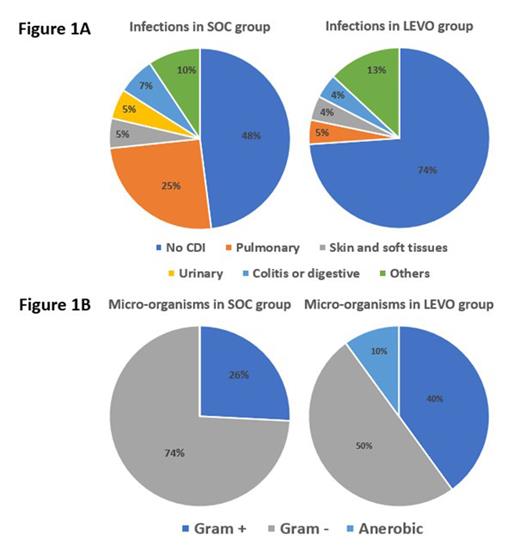
Results: The population included 264 patients, 71 received LEVO as primary antibioprophylaxis during 1 st course of VEN-AZA and 193 received no prophylaxis [antibiotherapy in case of febrile neutropenia, i.e. standard of care (SOC)]. The median age was 69 (range 20-87) years, 55.3% of patients were male, 48.5% of patients received VEN-AZA as front-line treatment and others 51.5% ≥ 2 nd line of treatment. Febrile neutropenia occurred in 32.4% of patients who received LEVO prophylaxis leading to a hospitalization of 100% of patient with antibiotherapy for a median of 10 days (range 2-45), as compared with 38.9% of those in SOC group leading to hospitalization of 73.3% of patient with antibiotherapy for a median of 10 days (range 1-31), (23 of 71 vs. 75 of 193; relative risk, 0.90; IC95% 0.74-1.10; P=0.33). Delay from day 1 VEN-AZA to febrile neutropenia was 23 (range 10-38) and 18 (range 7-44) days in LEVO and SOC groups (p=0.008), respectively. The LEVO group had a significantly lower rate of clinically documented infections (26.1% vs 52.0%, relative risk, 0.65; IC95% 0.46-0.91; P=0.03) (Figure 1A), but similar microbiologically documented infections (30.4% vs 32.0%) (Figure 1B). It is noteworthy that among patients receiving LEVO prophylaxis, no urinary tract infections were reported. The rate of lung infections was considerably reduced (5% vs 25%) as well as gram-negative bacterial infections (50% vs 74%), consistent with LEVO's broad spectrum, including non-fermenting micro-organisms. Anaerobia infections could be due to the lack of activity of LEVO on this bacterial spectrum. There was no difference in frequency of intensive care unit (ICU) hospitalization (1.4% vs 5.2%, P=0.29). No selection of extended spectrum beta-lactamase bacteria nor burden of fluoroquinolone resistance has been detected. Clostridium difficile colitis has been detected in 2 patients, both in SOC group. No adverse musculoskeletal events have been observed.
Conclusion: A short course of levofloxacin as primary prophylaxis in AML patients treated with VEN-AZA was well tolerated, seemed to have no impact on microbial resistance and was associated with a lower rate of clinically documented infection but not febrile neutropenia.
Disclosures
Recher:Jazz Pharmaceuticals: Other: Personal fees, Research Funding; Novartis: Other: Personal fees; Astellas: Other: Personal fees; BMS: Other: Personal fees, Research Funding; Amgen: Research Funding; Abbvie: Honoraria; Servier: Other: Personal fees; MaatPharma: Research Funding; IQVIA: Research Funding; Takeda: Other: Personal fees. Pigneux:Novartis: Honoraria; BMS: Membership on an entity's Board of Directors or advisory committees, Research Funding; Roche: Research Funding; Servier: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Support for attending meetings, Research Funding; Abbvie: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Support for attending meetings; Gilead: Honoraria; Jazz Pharmaceuticals: Honoraria, Membership on an entity's Board of Directors or advisory committees; Astellas: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Pfizer: Membership on an entity's Board of Directors or advisory committees. Bertoli:Jazz Pharmaceuticals: Honoraria, Other: Travel; Abbvie: Honoraria, Other: Travel; Astellas: Honoraria; Servier: Honoraria; BMS-Celgene: Honoraria; Novartis: Honoraria. Dumas:Astellas: Honoraria, Other: Research support for institution; Abbvie: Honoraria; BMS: Honoraria, Other: Research support for institution; Servier: Honoraria, Other: Research support for institution; Novartis: Honoraria, Other: Research support for institution; Daiichi-Sankyo: Honoraria, Other: Research support for institution; Janssen: Honoraria; Jazz pharmaceutical: Honoraria; Roche: Other: Research support for institution.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal